Abstract
Targeted i.v. busulfan dosing in pediatric non-malignant and malignant disorders undergoing HCT has been challenging due to the unpredictable PK of busulfan in young children. In 59 children with b-thalassemia undergoing HCT, we have previously reported that Bu TDM is feasible, and targeted IV Bu resulted in better EFS than untargeted oral bu in these patients (Balasubramanian et al., TCT 2019). However, the incidence of graft rejection was still high, probably due to age-related clearance of busulfan in very young children (Gaziev J et al. Blood. 2010).
Precision dosing models result in less inter-patient variability in systemic exposure and better treatment outcomes (Shukla P et al. Front Pharmacol. 2020). We carried out a pilot study of precision busulfan dosing in pediatric patients in collaboration with InsightRX(California, USA). We aimed to evaluate the performance of the InsightRX precision dosing model for busulfan in young patients undergoing HCT and compare with the incidence of mixed chimerism on days 30, 60, and 90 post HCT [> 5% recipient cells], the time to neutrophil and platelet engraftment and the incidence of regimen related toxicities.
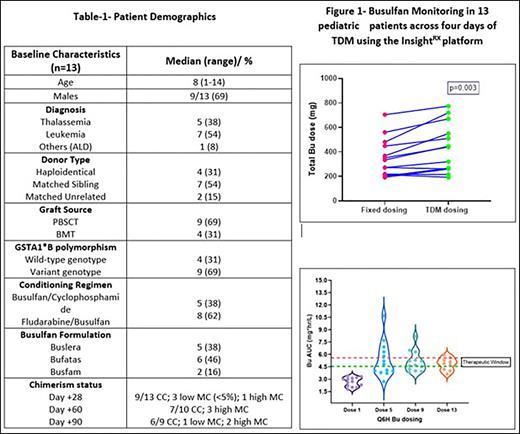
Thirteen patients were enrolled in the study from June 2021 to May 2022, and the demographics are listed in Table 1. Initial Bu dosing was based on EMA guidelines (Body weight-based dosing, Palmer J et al. BBMT. 2016). Busulfan levels were monitored after doses 1, 5, 9, and 13 using a validated LC-MS/MS method (Desire et al., 2013). The values were entered in the InsightRX TDM algorithm, and a cumulative AUC of 80-90mg.hr/L was targeted. The mean Bu dose per EMA guideline and the actual dose received after precision dosing was compared.
Based on InsightRX prediction, ten patients received higher cumulative Bu dosing, and 3 received lower cumulative Bu dosing compared to fixed weight-based dosing (Fig 1A). All patients had lower Bu AUC (<4.6 mg*hr/L) after the 1st dose, while at the end of the 13th dose, most patients achieved therapeutic Bu AUC (Median: 4.8 (3.6-5.8 mg*hr/L, Fig 1B). The interpatient variability in systemic exposure was only 12% with precision dosing, compared to 33% with the trapezoidal rule-based method (Balasubramanian et al., TCT 2019).
Irrespective of the busulfan formulation, GST genotype, and underlying disease, the total cumulative target AUC was achieved in all the patients; two patients rejected the graft (one rejected within day+90 and the other rejected late after day+90, both had unrelated donor source and lowered cumulative Bu AUC compared to the other patients), and one developed Sinusoidal obstruction syndrome (despite lower cumulative Bu AUC, therefore cannot be attributed to Bu exposure).
Targeting higher cumulative Bu exposure using a model-informed precision dosing software resulted in better HCT outcomes. Our study suggests the feasibility of improved precision of dosing of busulfan in young children undergoing HCT resulting in improved outcomes.
Disclosures
Hughes:InsightRX: Current Employment. Srivastava:Sanofi: Other: The study was supported by Sanofi, Research Funding; Pfizer: Research Funding; Roche: Research Funding; NovoNordisk: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal